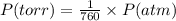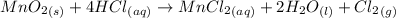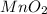Chemistry, 18.10.2019 21:00 lululoveee586
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, hcl(aq) , as described by the chemical equation mno2(s)+4hcl(aq)⟶mncl2(aq)+2h2o(l)+ cl2(g) how much mno2(s) should be added to excess hcl(aq) to obtain 255 ml cl2(g) at 25 °c and 725 torr ?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions

Biology, 27.07.2019 23:00

Social Studies, 27.07.2019 23:00

History, 27.07.2019 23:00

Physics, 27.07.2019 23:00






Mathematics, 27.07.2019 23:00





Business, 27.07.2019 23:00



History, 27.07.2019 23:00

Social Studies, 27.07.2019 23:00