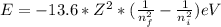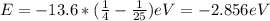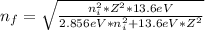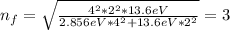Chemistry, 18.10.2019 18:30 gyexisromero10
When an excited electron in a hydrogen atom falls from =5 to =2, a photon of blue light is emitted. if an excited electron in an he+ ion falls from =4, which energy level must it fall to ) for blue light of a similar wavelength to be emitted?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
When an excited electron in a hydrogen atom falls from =5 to =2, a photon of blue light is emitted....
Questions


Mathematics, 07.10.2019 20:00







History, 07.10.2019 20:00


Chemistry, 07.10.2019 20:00

Mathematics, 07.10.2019 20:00







Mathematics, 07.10.2019 20:00