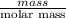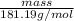You do an experiment in which you need 0.5 mole of tyrosine (c9h11no3) .
how many grams...

Chemistry, 07.10.2019 20:00 mamasmontoya
You do an experiment in which you need 0.5 mole of tyrosine (c9h11no3) .
how many grams must you weigh out?
65 g
90.5 g
181 g
272 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
Questions


Chemistry, 05.07.2019 16:30

Biology, 05.07.2019 16:30

Business, 05.07.2019 16:30


Mathematics, 05.07.2019 16:30


Spanish, 05.07.2019 16:30

Biology, 05.07.2019 16:30



Geography, 05.07.2019 16:30

Mathematics, 05.07.2019 16:30

Mathematics, 05.07.2019 16:30






History, 05.07.2019 16:30

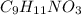 ) is 181.19 g/mol.
) is 181.19 g/mol.