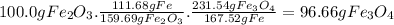calculate the weight of fe3o4 in 100.0g fe2o3.
given: molar mass of fe2o3 =159.69 g/mol...

Chemistry, 18.10.2019 01:00 CameronVand21
calculate the weight of fe3o4 in 100.0g fe2o3.
given: molar mass of fe2o3 =159.69 g/mol molar mass of fe3o4 = 231.54 g/mol hint: you need two conversion factors

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
Questions

Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


Physics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Arts, 18.03.2021 02:40

English, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

Spanish, 18.03.2021 02:40