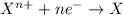As a result of the transfer of an electron from a less electronegative atom to a more electronegative atom,
a. the more electronegative atom is oxidized, and energy is released.
b. the more electronegative atom is reduced, and energy is consumed.
c. the more electronegative atom is oxidized, and energy is consumed.
d. the more electronegative atom is reduced, and energy is released.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1


Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
As a result of the transfer of an electron from a less electronegative atom to a more electronegativ...
Questions

Chemistry, 10.07.2019 19:30






English, 10.07.2019 19:30


Spanish, 10.07.2019 19:30

Chemistry, 10.07.2019 19:30

History, 10.07.2019 19:30


Arts, 10.07.2019 19:30

Mathematics, 10.07.2019 19:30






Mathematics, 10.07.2019 19:30