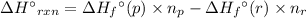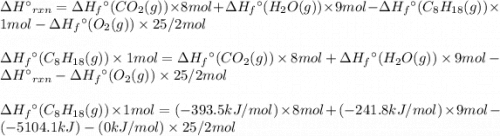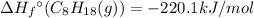Chemistry, 17.10.2019 03:20 mariahdelossantos031
For a particular isomer of c8h18, the combustion reaction produces 5104.1 kj of heat per mole of c8h18(g) consumed, under standard conditions. c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh ∘rxn=−5104.1 kj/mol what is the standard enthalpy of formation of this isomer of c8h18(g)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
For a particular isomer of c8h18, the combustion reaction produces 5104.1 kj of heat per mole of c8h...
Questions

Chemistry, 20.05.2020 04:02





Chemistry, 20.05.2020 04:02

Chemistry, 20.05.2020 04:02

Mathematics, 20.05.2020 04:02


Mathematics, 20.05.2020 04:02


Mathematics, 20.05.2020 04:02

Physics, 20.05.2020 04:02



History, 20.05.2020 04:03

Mathematics, 20.05.2020 04:03

Mathematics, 20.05.2020 04:03


Mathematics, 20.05.2020 04:03