
Chemistry, 15.10.2019 07:10 xxaurorabluexx
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 2.3 g of butane is mixed with 15.0 g of oxygen. calculate the minimum mass of butane that could be left over by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions

English, 02.12.2020 14:00




Mathematics, 02.12.2020 14:00

English, 02.12.2020 14:00

History, 02.12.2020 14:00



Mathematics, 02.12.2020 14:00



Biology, 02.12.2020 14:00

Arts, 02.12.2020 14:00

Mathematics, 02.12.2020 14:00



Business, 02.12.2020 14:00

English, 02.12.2020 14:00

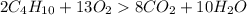
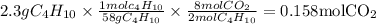
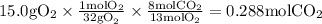
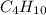 is the Limiting reactant and
is the Limiting reactant and 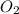 is the excess reactant
is the excess reactant
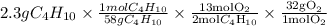
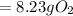 is the actual need
is the actual need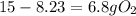 is left unreacted (Answer)
is left unreacted (Answer)

