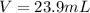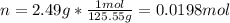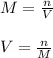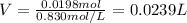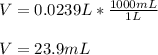Chemistry, 02.12.2020 14:00 isabelperez063
1. If 2.49 g of CuNO3 is dissolved in water to make a 0.830 M solution, what is the volume of the solution in milliliters?
2. How many moles of NaOH are present in 13.5 mL of 0.170 M NaOH?
3. Calculate the molarity of 0.650 mol of Na2S in 1.15 L of solution.
4. A student in lab needs to make a solution that is 7.00% by mass NaCl. If 145 g of NaCl is available, what mass of solution can be prepared?
5. Calculate the molarity of 24.1 g of MgS in 777 mL of solution.
6. A solution made by adding 16.3mL of methyl alcohol to enough water to give 541
mL of solution.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
1. If 2.49 g of CuNO3 is dissolved in water to make a 0.830 M solution, what is the volume of the so...
Questions

Business, 22.07.2019 10:00


Mathematics, 22.07.2019 10:00

Social Studies, 22.07.2019 10:00



Health, 22.07.2019 10:00

Mathematics, 22.07.2019 10:00

Mathematics, 22.07.2019 10:00


Chemistry, 22.07.2019 10:00

Biology, 22.07.2019 10:00


English, 22.07.2019 10:00



Mathematics, 22.07.2019 10:00


Arts, 22.07.2019 10:00

Mathematics, 22.07.2019 10:00