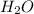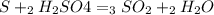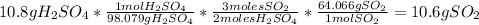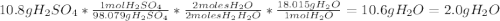Chemistry, 15.10.2019 04:00 johnnyboy41706
For each of the following unbalanced equations, calculate how many grams of each product would be produced by complete reaction of 10.8 g of the second reactant. (a) s(s) + h2so4(aq) → so2(g) + h2o(l) so2 webassign will check your answer for the correct number of significant figures. g h2o webassign will check your answer for the correct number of significant figures. g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
For each of the following unbalanced equations, calculate how many grams of each product would be pr...
Questions

Mathematics, 23.09.2021 01:30

Mathematics, 23.09.2021 01:30




English, 23.09.2021 01:30

Social Studies, 23.09.2021 01:30

Spanish, 23.09.2021 01:30


Mathematics, 23.09.2021 01:30




Biology, 23.09.2021 01:30

Mathematics, 23.09.2021 01:30

Mathematics, 23.09.2021 01:30

Mathematics, 23.09.2021 01:40


Mathematics, 23.09.2021 01:40

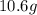 of
of 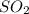
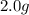 of
of