
Physics, 23.09.2021 01:30 fseftrwer2522
Look at the emission spectrum that you drew for hydrogen. The wavelengths of the spectral lines correspond to emissions of photons by electrons in excited states transitioning to a new quantum level. The wavelengths for the most prominent 3 spectral lines of hydrogen are:
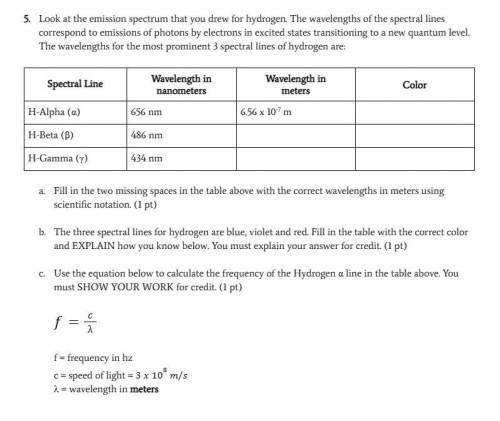

Answers: 3


Another question on Physics

Physics, 21.06.2019 16:00
28. a stone is projected at a cliff of height h with an initial speed of 42.0 mls directed at angle θ0 = 60.0° above the horizontal. the stone strikes at a, 5.50 s after launching. find (a) the height h of the cliff, (b) the speed of the stone just before impact at a, and (c) the maximum height h reached above the grou
Answers: 1

Physics, 22.06.2019 15:00
When is a current produced? when the terminals of an electrochemical cell are connected by a wire if the electric circuit is opened in an electrochemical cell if the electrolyte is removed from an electrochemical cell when the electrodes are reversed in an electrochemical cell
Answers: 1

Physics, 22.06.2019 16:00
At what time will the interfering waves have an amplitudeof zero?
Answers: 1

Physics, 22.06.2019 17:30
Four point charges each having charge q are located at the corners of a square having sides of length a. find symbolic expressions for the following. (a) the total electric potential at the center of the square due to the four charges (use any variable or symbol stated above along with the following as necessary: ke.) vtotal= (b) the work required to bring a fifth charge p from infinity to the center of the square (use any variable or symbol stated above along with the following as necessary: w=
Answers: 2
You know the right answer?
Look at the emission spectrum that you drew for hydrogen. The wavelengths of the spectral lines corr...
Questions






Biology, 28.01.2020 15:04

Mathematics, 28.01.2020 15:04

English, 28.01.2020 15:04




Social Studies, 28.01.2020 15:04


Physics, 28.01.2020 15:04

Mathematics, 28.01.2020 15:04


Business, 28.01.2020 15:04

Physics, 28.01.2020 15:04


Mathematics, 28.01.2020 15:04



