
Chemistry, 14.10.2019 17:30 alexthebest3976
Which of the following chemical equations does not correspond to a standard molar enthalpy of formation? a. mg(s) + c(s) + 3/2 o_2(g) rightarrow mgco_3(s) b. c(s) + 1/2 o_2(g) rightarrow co(g) c. n_2(g) + o_2(g) rightarrow 2 no(g) d. n_2(g) + 2 o_2(g) rightarrow n_2o_4(g) e. h_2(g)+l/2 o_2(g) rightarrow h_2o(l)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3
You know the right answer?
Which of the following chemical equations does not correspond to a standard molar enthalpy of format...
Questions




Mathematics, 08.11.2021 14:00


English, 08.11.2021 14:00



Chemistry, 08.11.2021 14:00





Mathematics, 08.11.2021 14:00

Biology, 08.11.2021 14:00


History, 08.11.2021 14:00

Mathematics, 08.11.2021 14:00


Health, 08.11.2021 14:00

 + O₂
+ O₂ → 2NO
→ 2NO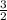 O
O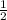 O₂
O₂


