
Chemistry, 09.10.2019 02:00 tylerineedhelp
A. the half-life for a first order reaction in 49 minutes. what is the rate constant? b. for a second order reaction, the half-life is 726 seconds. starting with a concentration of 0.600 m, how long will it take for the concentration to decrease to 0.150 m? c. the half-life for a first order reaction is 2768 years. starting with a concentration of 0.345 m, what will the concentration be after 11,072 years? d. the half-life for first order reaction a is 75 minutes. the half-life for a different first order reaction b is 322 minutes. which reaction has the faster rate? (a or b) which reaction has the larger rate constant, k? (a or b)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:50
What is the specific heat of a substance that absorbs 2.5×10^3 joules of heat when a sample of 1.0 ×10^4g of the substance increases in temperature from 10°c to 70°c?
Answers: 2

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
A. the half-life for a first order reaction in 49 minutes. what is the rate constant? b. for a secon...
Questions






Computers and Technology, 19.05.2021 19:00

Chemistry, 19.05.2021 19:00


Mathematics, 19.05.2021 19:00

Mathematics, 19.05.2021 19:00



Chemistry, 19.05.2021 19:00

Biology, 19.05.2021 19:00


Mathematics, 19.05.2021 19:00




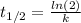 (1) As half.life is 49 min. Rate constant -k- is: 0,014 min⁻¹
(1) As half.life is 49 min. Rate constant -k- is: 0,014 min⁻¹

