Chemistry, 08.10.2019 05:10 plantkiana677oxa6hk
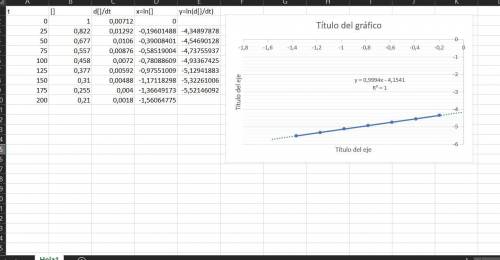
The data below show the concentration of n2o5 versus time for the following reaction: n2o5(g)→no3(g)+no2(g) time (s) [n2o5] (m) 0 1.000 25 0.822 50 0.677 75 0.557 100 0.458 125 0.377 150 0.310 175 0.255 200 0.210 part a determine the order of the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
The data below show the concentration of n2o5 versus time for the following reaction: n2o5(g)→no3(g...
Questions


Mathematics, 29.04.2021 09:00


Mathematics, 29.04.2021 09:00


Mathematics, 29.04.2021 09:00

History, 29.04.2021 09:00

History, 29.04.2021 09:00


History, 29.04.2021 09:00

Mathematics, 29.04.2021 09:00

Mathematics, 29.04.2021 09:00

Health, 29.04.2021 09:00

Mathematics, 29.04.2021 09:00

Mathematics, 29.04.2021 09:00





![\frac{d[N_2O_5]}{dt} =-k[N_2O_5]^n](/tpl/images/0299/3705/c658d.png)
![ln(\frac{d[N_2O_5]}{dt})=ln(k)+nln([N_2O_5])](/tpl/images/0299/3705/dd5aa.png)