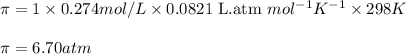Chemistry, 28.09.2019 01:30 taylorclmna
The nonvolatile, nonelectrolyte estrogen (estradiol), c18h24o2 (272.40 g/mol), is soluble in chloroform chcl3.
calculate the osmotic pressure generated when 13.5 grams of estrogen are dissolved in 181 ml of a chloroform solution at 298 k.
the molarity of the solution is ? m.
the osmotic pressure of the solution is ? atmospheres.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
The nonvolatile, nonelectrolyte estrogen (estradiol), c18h24o2 (272.40 g/mol), is soluble in chlorof...
Questions


Health, 30.01.2020 08:49

Mathematics, 30.01.2020 08:49


Mathematics, 30.01.2020 08:49

Mathematics, 30.01.2020 08:50


Mathematics, 30.01.2020 08:50




History, 30.01.2020 08:50


English, 30.01.2020 08:50


History, 30.01.2020 08:50

Mathematics, 30.01.2020 08:50

Physics, 30.01.2020 08:50


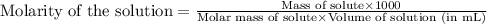
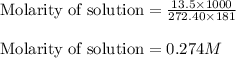
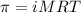
 = osmotic pressure of the solution = ?
= osmotic pressure of the solution = ?