
Chemistry, 24.09.2019 03:20 haydencheramie
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen molecules) into the engine. consider a car that is using 100% ethanol, c2h5oh, as fuel. if your engine intakes 4.73 l of air per minute at 1.00 atm and 25ºc, what is the maximum volume of ethanol (0.789 g/ml) that can be burned per minute? hint: you can ignore the "per minute" information because both the ethanol and air are being quantified per minute. enter your answer to three significant figures in units of ml.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen...
Questions

Mathematics, 26.01.2021 06:00

English, 26.01.2021 06:00

Mathematics, 26.01.2021 06:00


Mathematics, 26.01.2021 06:00




Mathematics, 26.01.2021 06:00


Computers and Technology, 26.01.2021 06:00

Mathematics, 26.01.2021 06:00

Biology, 26.01.2021 06:00



Mathematics, 26.01.2021 06:00

French, 26.01.2021 06:00


Mathematics, 26.01.2021 06:00

Computers and Technology, 26.01.2021 06:00

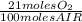 = 0,0406 moles O₂
= 0,0406 moles O₂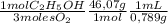 = 0,895 mL of ethanol per minute
= 0,895 mL of ethanol per minute

