
Polonium- 210 , po210 , decays to lead- 206 , pb206 , by alpha emission according to the equation po84210⟶pb82206+he24 if the half-life, /2 , of po210 is 138.4 days , calculate the mass of pb206 produced from a 561.0 mg sample of polonium(iv) chloride, pocl4 , that is left untouched for 333.8 days . assume that the only polonium isotope present in the sample is the po210 isotope. the isotopic molar masses of po210 is 209.98 g/mol and pb206 is 205.97 g/mol .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
Polonium- 210 , po210 , decays to lead- 206 , pb206 , by alpha emission according to the equation po...
Questions

History, 09.04.2021 03:40

History, 09.04.2021 03:40


Mathematics, 09.04.2021 03:40


Chemistry, 09.04.2021 03:40




Mathematics, 09.04.2021 03:40


Spanish, 09.04.2021 03:40



Mathematics, 09.04.2021 03:40

Health, 09.04.2021 03:40




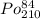 ⟶
⟶ 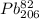 +
+ 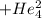
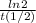
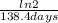 =0,005008 days^(-1)
=0,005008 days^(-1)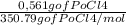 = 0,001599 mol of PoCl4
= 0,001599 mol of PoCl4

