Prob.: consider the combustion of butane (c4h10):
2c4h10(g) + 13o2(g) ==> 8co2(g) + 10 h...

Chemistry, 14.09.2019 00:30 zabomoxx5ll
Prob.: consider the combustion of butane (c4h10):
2c4h10(g) + 13o2(g) ==> 8co2(g) + 10 h2o(l)
in a particular reaction, 5.0 moles of c4h10 are reacted
withan excess of o2. calculate the number of moles of co2
formed.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
Questions



Computers and Technology, 20.12.2019 18:31


History, 20.12.2019 18:31

Biology, 20.12.2019 18:31

Mathematics, 20.12.2019 18:31



English, 20.12.2019 18:31


English, 20.12.2019 18:31




Mathematics, 20.12.2019 18:31


History, 20.12.2019 18:31


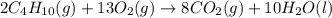
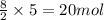 of carbon dioxide.
of carbon dioxide.

