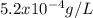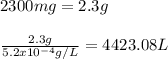Chemistry, 10.09.2019 20:30 haithjamel
How many liters of softened water, containing a sodium concentration of 5.2×10−2 % sodium by mass, have to be consumed to exceed the fda recommendation? (assume a water density of 1.0 g/ml.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Pauling and lewis questioned the extreme definitions of bonds. they wondered if bonds might be described somewhere in between the two extremes (covalent and ionic). on the basis of experimental data,pauling confirmed that bonds could be ionic, covalent, and for those, in between, exhibit a degree of ionic character. he theorized that the major factor was how strongly the atoms in the bond attracted the electrons. pauling called this factor - the tendency of an atom to attract electrons in a bond.
Answers: 2

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1
You know the right answer?
How many liters of softened water, containing a sodium concentration of 5.2×10−2 % sodium by mass, h...
Questions

Mathematics, 02.12.2019 17:31




English, 02.12.2019 17:31





Geography, 02.12.2019 17:31



Social Studies, 02.12.2019 17:31



Mathematics, 02.12.2019 17:31




Mathematics, 02.12.2019 17:31