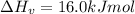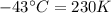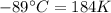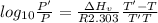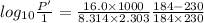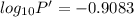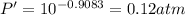Chemistry, 10.09.2019 01:30 natetheman7740
The enthalpy of vaporization of substance x is 16.0kj mol and its normal boiling point is −43.°c. calculate the vapor pressure of x at −89.°c. round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
The enthalpy of vaporization of substance x is 16.0kj mol and its normal boiling point is −43.°c. ca...
Questions




Mathematics, 01.10.2019 00:10


Social Studies, 01.10.2019 00:10

English, 01.10.2019 00:10






Mathematics, 01.10.2019 00:10

Mathematics, 01.10.2019 00:10

Biology, 01.10.2019 00:10

Chemistry, 01.10.2019 00:10




Social Studies, 01.10.2019 00:10

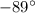 = 0.12 atm
= 0.12 atm