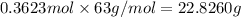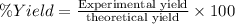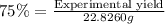Chemistry, 09.09.2019 17:20 victoria1831
If the percent yield for the following reaction is 75.0%, and 25.0 g of no₂ are consumed in the reaction, how many grams of nitric acid, hno₃(aq) are produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1
You know the right answer?
If the percent yield for the following reaction is 75.0%, and 25.0 g of no₂ are consumed in the reac...
Questions


History, 16.07.2019 11:30



Mathematics, 16.07.2019 11:30


Biology, 16.07.2019 11:30

Social Studies, 16.07.2019 11:30

Social Studies, 16.07.2019 11:30



Health, 16.07.2019 11:30



Mathematics, 16.07.2019 11:30



Chemistry, 16.07.2019 11:30


History, 16.07.2019 11:30

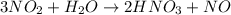
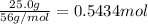
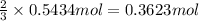 of nitric acid.
of nitric acid.