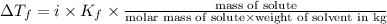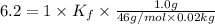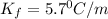Chemistry, 06.09.2019 03:30 ineedtopeebeforethec
Pure nitrobenzene freezes at 5.67 c. when 1.0g of ethanol (c2h6o) is mixed with 20.0g nitrobenzene, the freeze point drops to –0.53 c. what is the freezing-point depression constant (kf) of nitrobenzene?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
Pure nitrobenzene freezes at 5.67 c. when 1.0g of ethanol (c2h6o) is mixed with 20.0g nitrobenzene,...
Questions

Mathematics, 17.06.2020 04:57


Computers and Technology, 17.06.2020 04:57


English, 17.06.2020 04:57




Mathematics, 17.06.2020 04:57


Health, 17.06.2020 04:57


Physics, 17.06.2020 04:57



Mathematics, 17.06.2020 04:57




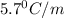
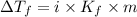
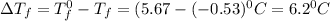 = Depression in freezing point
= Depression in freezing point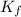 = freezing point constant = ?
= freezing point constant = ?