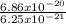Chemistry, 02.09.2019 21:30 graciegirl6662
Water standing in the open at 29.2°c evaporates because of the escape of some of the surface molecules. the heat of vaporization (548 cal/g) is approximately equal to εn, where ε is the average energy of the escaping molecules and n is the number of molecules per gram.
(a) find ε.
(b) what is the ratio of ε to the average kinetic energy of h2o molecules, assuming the latter is related to temperature in the same way as it is for gases?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Water standing in the open at 29.2°c evaporates because of the escape of some of the surface molecul...
Questions

Computers and Technology, 06.06.2021 16:20

English, 06.06.2021 16:20



Business, 06.06.2021 16:20

Physics, 06.06.2021 16:20

English, 06.06.2021 16:30

Chemistry, 06.06.2021 16:30

Mathematics, 06.06.2021 16:30

English, 06.06.2021 16:30

Mathematics, 06.06.2021 16:30

Arts, 06.06.2021 16:30


Biology, 06.06.2021 16:30



History, 06.06.2021 16:30

Business, 06.06.2021 16:30


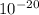 J
J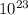 molecules, so n will be:
molecules, so n will be: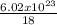
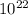 molecules/g
molecules/g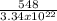
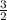 kT
kT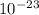 J/K and T = 29.2ºC + 273 = 302.2 K
J/K and T = 29.2ºC + 273 = 302.2 K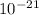 J
J