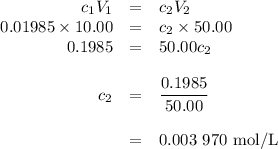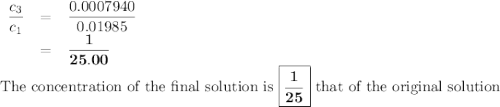Chemistry, 31.08.2019 03:10 dsaefong00
the molar concentration (m) of a solution prepared by dissolving 0.2362g of cr(no3)3 in a 50-ml volumetric flask is 0.01985m, where the molecular weight for cr(no3)3 = 238.01g/mol.
a. suppose you want to prepare another solution containing chromium nitrate that is 25 times less concentrated than the one prepared above. given a choice of 10-ml and 5-ml pipets and 50-ml and 100-ml volumetric flasks, explain how you would proceed in preparing the new diluted solution. in addition, calculate the concentration for the new diluted solution. show all work. your final value should have the correct unit and number of significant figures. hint: you will most likely need two dilution steps in order to obtain the desired concentration. note: you may not reuse the same pipet or combine different pipets within the same dilution step. you may reuse the pipet and/or volumetric flask in the different dilution step.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
the molar concentration (m) of a solution prepared by dissolving 0.2362g of cr(no3)3 in a 50-ml volu...
Questions

Biology, 20.09.2020 07:01



Mathematics, 20.09.2020 07:01

Chemistry, 20.09.2020 07:01

Computers and Technology, 20.09.2020 07:01





English, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

English, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

Chemistry, 20.09.2020 07:01

Chemistry, 20.09.2020 07:01