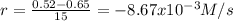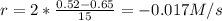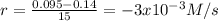a + b --> 2c

Chemistry, 30.08.2019 23:30 angsoccer02
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
concentration time data at t = 30oc
time (s) [a] (mol l-1)
0 0.65
15 0.52
30 0.35
45 0.28
60 0.22
75 0.14
90 0.095
(i) calculate the average rate of consumption of a in the first 15 seconds of reaction.
(ii) calculate the average rate of production of c in the first 15 seconds of reaction.
(iii) calculate the average rate of consumption of a in the last 15 seconds of the reaction.
(iv) explain the difference between the rates of consumption calculated in (i) and that in (iii)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
a + b --> 2c
Questions

English, 19.08.2019 21:30


Biology, 19.08.2019 21:30




History, 19.08.2019 21:30



World Languages, 19.08.2019 21:30


Mathematics, 19.08.2019 21:30

Social Studies, 19.08.2019 21:30

Social Studies, 19.08.2019 21:30

Mathematics, 19.08.2019 21:30


Mathematics, 19.08.2019 21:30

English, 19.08.2019 21:30

Mathematics, 19.08.2019 21:30

![r=\frac{\Delta [Concentration]}{\Delta t}](/tpl/images/0213/3224/c01cf.png)