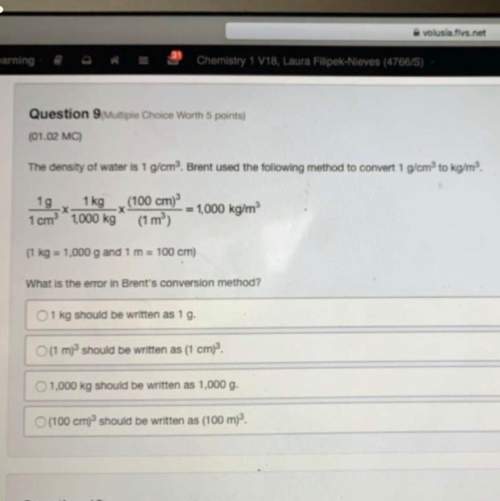
Chemistry, 29.08.2019 20:20 jessnolonger
Carbon, hydrogen and ethane each burn exothermically in an excess of air. ahⓡ =-393.7 kj mol. c(s) + o2(g) → co2(g) h2(g) + % o2(g) → h20(1) czha(g) + 302() → 2co2(g) + 2h2o(1) ah®=-285.9 kj mol ah =-1411.0 kj moll. use the data to calculate the standard enthalpy change of formation, ah in kj mol'', of ethene at 298 k 2c(s) + 2h2(g) → c2h4(g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Carbon, hydrogen and ethane each burn exothermically in an excess of air. ahⓡ =-393.7 kj mol. c(s) +...
Questions

History, 27.11.2019 20:31




Biology, 27.11.2019 20:31



Mathematics, 27.11.2019 20:31

Mathematics, 27.11.2019 20:31




Mathematics, 27.11.2019 20:31

History, 27.11.2019 20:31

Mathematics, 27.11.2019 20:31




Social Studies, 27.11.2019 20:31

History, 27.11.2019 20:31

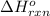 for the reaction is 51.8 kJ.
for the reaction is 51.8 kJ.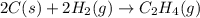
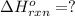
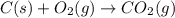
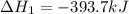 ( × 2)
( × 2)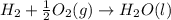
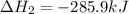 ( × 2)
( × 2)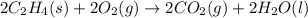

![\Delta H^o_{rxn}=[2\times \Delta H_1]+[2\times \Delta H_2]+[1\times (-\Delta H_3)]](/tpl/images/0209/7515/e45ac.png)
![\Delta H^o_{rxn}=[(2\times (-393.7))+(2\times (-285.9))+(1\times -(-1411))]=51.8kJ](/tpl/images/0209/7515/5c6c6.png)