Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Lithium and nitrogen react to produce lithium nitride: 6li + n2 --> 2li3n how many moles of n2...
Questions

Mathematics, 20.03.2020 02:02

Biology, 20.03.2020 02:02



Mathematics, 20.03.2020 02:02

English, 20.03.2020 02:02

English, 20.03.2020 02:02


Law, 20.03.2020 02:02


History, 20.03.2020 02:02



English, 20.03.2020 02:02

History, 20.03.2020 02:02




Mathematics, 20.03.2020 02:02

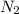 that is required for reacting with the 0.500 mol of Lithium is 0.0833 moles.
that is required for reacting with the 0.500 mol of Lithium is 0.0833 moles.