
Chemistry, 20.03.2020 02:02 cookie42087
2.00 moles of CO2 and 1.50 moles of H2 are placed into a rigid 5.00-L container and they react according to the equation CO2(g) + H2(g) ⇄ CO(g) + H2O(g) K = 2.50 What will be the concentration of carbon monoxide when equilibrium is reached? 0.191 M 0.091 M 0.209 M (Your correct answer) 0.913 M 1.05 M

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2


You know the right answer?
2.00 moles of CO2 and 1.50 moles of H2 are placed into a rigid 5.00-L container and they react accor...
Questions



Mathematics, 24.04.2020 19:36



Computers and Technology, 24.04.2020 19:36

Mathematics, 24.04.2020 19:36





Biology, 24.04.2020 19:36


Physics, 24.04.2020 19:36

World Languages, 24.04.2020 19:36

English, 24.04.2020 19:36



Mathematics, 24.04.2020 19:36

Health, 24.04.2020 19:36

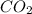 = 2.00 mole
= 2.00 mole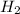 = 1.50 mole
= 1.50 mole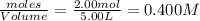
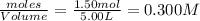
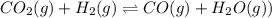
![K_c=\frac{[CO]\times [H_2O]}{[H_2]\times [CO_2]}](/tpl/images/0555/1888/1dc80.png)
![2.50=\frac{[x]\times [x]}{[0.300-x]\times [0.400-x]}](/tpl/images/0555/1888/54f3c.png)
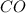 at equilibrium = x M = 0.209 M
at equilibrium = x M = 0.209 M

