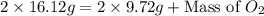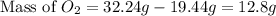Chemistry, 28.08.2019 17:20 chloesmolinski0909
The law of conservation of mass states that mass is neither created nor destroyed during a chemical reaction. this can be gleaned from the third postulate in dalton's series. magnesium oxide decomposes into magnesium and oxygen. if 16.12 g of magnesium oxide decomposes to form 9.72 g of magnesium, what mass of oxygen gas is also released in the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g
Answers: 2

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
The law of conservation of mass states that mass is neither created nor destroyed during a chemical...
Questions



Mathematics, 17.07.2019 12:00




Mathematics, 17.07.2019 12:00

Mathematics, 17.07.2019 12:00


History, 17.07.2019 12:00

History, 17.07.2019 12:00


Health, 17.07.2019 12:00


Mathematics, 17.07.2019 12:00

History, 17.07.2019 12:00

Mathematics, 17.07.2019 12:00


Mathematics, 17.07.2019 12:00

Mathematics, 17.07.2019 12:00

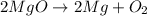
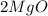 = Total mass of
= Total mass of 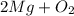
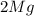 + Mass of
+ Mass of 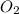
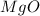 = 16.12 grams
= 16.12 grams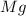 = 9.72 grams
= 9.72 grams