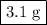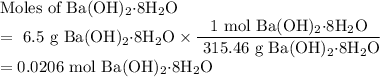Chemistry, 27.08.2019 00:20 insaneshootermo
One of relatively few reactions that takes place directly between two solids at room temperature is ba(oh)2 · 8h2o + ammonium thiocyanate --> barium thiocyanate + water + ammonia in this equation, the · 8h2o in ba(oh)2 · 8h2o indicates the presence of eight water molecules. this compound is called barium hydroxide octahydrate. what mass of ammonium thiocyanate must be used if it is to react completely with 6.5 g barium hydroxide octahydrate?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
One of relatively few reactions that takes place directly between two solids at room temperature is...
Questions


Mathematics, 31.01.2020 07:50

Mathematics, 31.01.2020 07:50

Mathematics, 31.01.2020 07:50


Mathematics, 31.01.2020 07:50

Computers and Technology, 31.01.2020 07:50

Social Studies, 31.01.2020 07:50

Mathematics, 31.01.2020 07:50

History, 31.01.2020 07:50

Mathematics, 31.01.2020 07:50


History, 31.01.2020 07:50

Biology, 31.01.2020 07:50

Mathematics, 31.01.2020 07:50



Mathematics, 31.01.2020 07:50

Mathematics, 31.01.2020 07:50