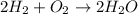Chemistry, 31.01.2020 07:50 sbhunsaker9025
Which of the following is true about the mass of the reactants in a chemical reaction? a. the total mass of the reactants in a chemical reaction will never be equal to the total mass of the products. b. the total mass of the reactants in a chemical reaction will be significantly more than the total mass of the products. c. the total mass of the reactants in a chemical reaction is conserved and will be equal to the total original mass of the products. d. the total mass of the reactants in a chemical reaction will be significantly less than the total mass of the products.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
Which of the following is true about the mass of the reactants in a chemical reaction? a. the total...
Questions

Mathematics, 24.03.2020 06:26

Mathematics, 24.03.2020 06:27







History, 24.03.2020 06:27

Mathematics, 24.03.2020 06:27

Biology, 24.03.2020 06:27


Mathematics, 24.03.2020 06:27

Mathematics, 24.03.2020 06:27


Biology, 24.03.2020 06:27