
Chemistry, 26.08.2019 18:00 juan01sebastian00
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen molecules and six hydrogen molecules in a closed container. assuming the reaction goes to completion, what will the final product mixture be?
a. number of nh3 molecules
b. number of n2 molecules
c. number of h2 molecules
which of the following equations best represents this reaction?
a. 42 n2 + 6 h2 4 nh3
b. 6 n2 + 6 h2 4 nh3 + 4 n2
c. n + 3 h2 nh3
d. n2 + 3 h2 2 nh3
e. n2 + h2 nh3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1


You know the right answer?
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen mole...
Questions

English, 10.06.2020 23:57



Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57


Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57



Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57




Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57

History, 10.06.2020 23:57

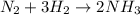
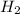 react completely with 1 molecule of
react completely with 1 molecule of 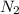 and produce 2 molecules of
and produce 2 molecules of 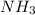 .
.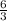 or 2 molecules of
or 2 molecules of  or 4 molecules of
or 4 molecules of 

