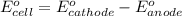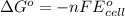Chemistry, 22.08.2019 21:30 trosclairozlynn02
Using the following standard reduction potentials, fe3+(aq) + e- → fe2+(aq) e° = +0.77 v ni2+(aq) + 2 e- → ni(s) e° = -0.23 v calculate the standard cell potential for the galvanic cell reaction given below, and determine whether or not this reaction is spontaneous under standard conditions. ni2+(aq) + 2 fe2+(aq) → 2 fe3+(aq) + ni(s)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
Using the following standard reduction potentials, fe3+(aq) + e- → fe2+(aq) e° = +0.77 v ni2+(aq) +...
Questions

English, 28.09.2019 12:20

Physics, 28.09.2019 12:20


History, 28.09.2019 12:20

Social Studies, 28.09.2019 12:20

Mathematics, 28.09.2019 12:20






Biology, 28.09.2019 12:20




Mathematics, 28.09.2019 12:20



Health, 28.09.2019 12:20

History, 28.09.2019 12:20

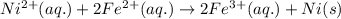
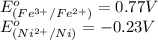
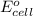 of the reaction, we use the equation:
of the reaction, we use the equation: