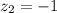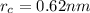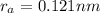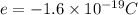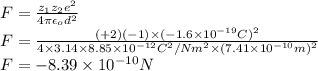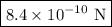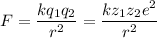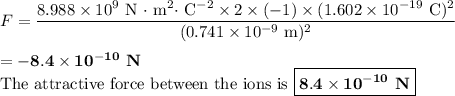Chemistry, 20.08.2019 05:30 JocelynC24
The atomic radii of a divalent cation and a monovalent anion are 0.62 nm and 0.121 nm, respectively. (a) calculate the force of attraction between these two ions at their equilibrium interionic separation (i. e., when the ions just touch one another).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this solution?
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2
You know the right answer?
The atomic radii of a divalent cation and a monovalent anion are 0.62 nm and 0.121 nm, respectively....
Questions

Computers and Technology, 01.12.2020 23:10


Mathematics, 01.12.2020 23:10

Biology, 01.12.2020 23:10

Social Studies, 01.12.2020 23:10

English, 01.12.2020 23:10

Biology, 01.12.2020 23:10

Mathematics, 01.12.2020 23:10


Mathematics, 01.12.2020 23:10

Biology, 01.12.2020 23:10

Mathematics, 01.12.2020 23:10



Physics, 01.12.2020 23:10





 .
.