

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
The complete combustion of octane, c8h18, a component of gasoline, proceeds as follows: 2c8h18(l)+2...
Questions


History, 06.02.2022 21:10

Mathematics, 06.02.2022 21:10



Mathematics, 06.02.2022 21:10


Mathematics, 06.02.2022 21:10

Social Studies, 06.02.2022 21:10

English, 06.02.2022 21:10


Physics, 06.02.2022 21:10






Arts, 06.02.2022 21:10

English, 06.02.2022 21:10

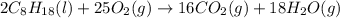
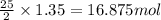 of oxygen are needed.
of oxygen are needed.


