
Chemistry, 08.08.2019 05:30 katier9407
Nernst equation – for the following reaction:
mn (s) + cr3+ (aq) ? mn2+ (aq) + cr (s)
(a) write the three balanced equations (each half-cell and the overall):
(b) what is the standard cell potential (e°cell) for this mn/cr cell?
(c) what will be the cell potential when [mn2+] = 0.20 m and [cr3+] = 1.0 × 10-4 m ?
(d) what will be the cell potential when [mn2+] = 1.0 × 10-4 m and [cr3+] = 0.20 m ?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1


Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1
You know the right answer?
Nernst equation – for the following reaction:
mn (s) + cr3+ (aq) ? mn2+ (aq) + cr (s)
...
mn (s) + cr3+ (aq) ? mn2+ (aq) + cr (s)
...
Questions





Social Studies, 28.04.2021 20:40

Mathematics, 28.04.2021 20:40



History, 28.04.2021 20:40






Mathematics, 28.04.2021 20:40



Chemistry, 28.04.2021 20:40


Mathematics, 28.04.2021 20:40


 ( × 3)
( × 3) ( × 2)
( × 2)
 of the reaction, we use the equation:
of the reaction, we use the equation: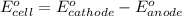

![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]}{[Cr^{3+}]}](/tpl/images/0173/1570/b6936.png)
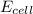 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[Cr^{3+}]=1.0\times 10^{-4}M](/tpl/images/0173/1570/252be.png)
![[Mn^{2+}]=0.20M](/tpl/images/0173/1570/1716f.png)

![[Cr^{3+}]=0.20M](/tpl/images/0173/1570/cae64.png)
![[Mn^{2+}]=1.0\times 10^{-4}M](/tpl/images/0173/1570/83dbd.png)



