
Chemistry, 06.08.2019 00:30 tybreyonnaHco7855
In the haber process for ammonia synthesis, k " 0.036 for n 2 (g) ! 3 h 2 (g) ∆ 2 nh 3 (g) at 500. k. if a 2.0-l reactor is charged with 1.42 bar of n 2 and 2.87 bar of h 2 , what will the equilibrium partial pressures in the mixture be?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
In the haber process for ammonia synthesis, k " 0.036 for n 2 (g) ! 3 h 2 (g) ∆ 2 nh 3 (g) at 500....
Questions

Mathematics, 27.08.2019 17:40


History, 27.08.2019 17:40




Geography, 27.08.2019 17:50


Mathematics, 27.08.2019 17:50

Mathematics, 27.08.2019 17:50



English, 27.08.2019 17:50

Biology, 27.08.2019 17:50



Mathematics, 27.08.2019 17:50



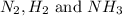 at equilibrium are, 1.133, 2.009, 0.574 bar respectively. The total pressure at equilibrium is, 3.716 bar
at equilibrium are, 1.133, 2.009, 0.574 bar respectively. The total pressure at equilibrium is, 3.716 bar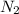 = 1.42 bar
= 1.42 bar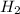 = 2.87 bar
= 2.87 bar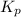 = 0.036
= 0.036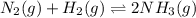
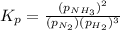
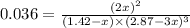
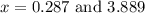
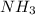 at equilibrium = 2x = 2 × 0.287 = 0.574 bar
at equilibrium = 2x = 2 × 0.287 = 0.574 bar

