

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
Determine the value of kc for the following reaction if the equilibrium concentrations are as follow...
Questions

English, 29.07.2021 17:50


History, 29.07.2021 17:50


Mathematics, 29.07.2021 17:50

Physics, 29.07.2021 17:50

Mathematics, 29.07.2021 17:50

Mathematics, 29.07.2021 17:50

History, 29.07.2021 17:50



Mathematics, 29.07.2021 17:50



Mathematics, 29.07.2021 17:50

Geography, 29.07.2021 17:50

Biology, 29.07.2021 17:50


Mathematics, 29.07.2021 17:50

Physics, 29.07.2021 17:50

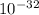
![K_{c} =\frac{[HI]^{2}[Cl_{2}] }{[HCl]^{2}}=\frac{[5.6*10^{-16} ]^{2} [0.0019]}{[0.13]^{2} }=3.53*10^{-32}](/tpl/images/0149/3443/d62e7.png)


