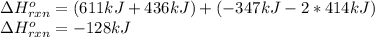Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3


Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Use the bond energies provided to estimate δh°rxn for the reaction below. c2h4(g) + h2(g) → c2h6(g)...
Questions

Geography, 25.03.2021 19:20




History, 25.03.2021 19:20


Mathematics, 25.03.2021 19:20



Mathematics, 25.03.2021 19:20

Mathematics, 25.03.2021 19:20

Mathematics, 25.03.2021 19:20





English, 25.03.2021 19:20



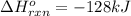
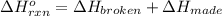
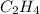 a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain:
a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain: