
Chemistry, 08.07.2019 18:10 Shadow0202
The combination of coke and steam produces a mixture called coal gas, which can be used as a fuel or as a starting material for other reactions. if we assume coke can be represented by graphite, the equation for the production of coal gas is
2c(s)+2h2o(> ch4(g)+co2(g)
determine the standard enthalpy change for this reactionf rom the following standard enthalpies of reactions:
c(s)+h2o(> co(g)+h2(g) delta h=131.3 kj
co(g)+h2o(> co2(g)+h2(g) delta h=-41.2 kj
ch4(g)+h2o(> 3h2(g)+co(g) delta h=206.1 kj

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1


Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
You know the right answer?
The combination of coke and steam produces a mixture called coal gas, which can be used as a fuel or...
Questions














Computers and Technology, 07.09.2019 03:10






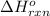 for the reaction is 15.3 kJ.
for the reaction is 15.3 kJ.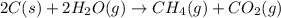
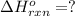
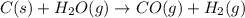
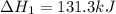 ( × 2)
( × 2)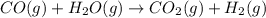

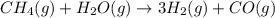
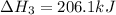
![\Delta H^o_{rxn}=[2\times \Delta H_1]+[1\times \Delta H_2]+[1\times (-\Delta H_3)]](/tpl/images/0066/4813/d71c9.png)
![\Delta H^o_{rxn}=[(2\times (131.3))+(1\times (-41.2))+(1\times (-206.1))]=15.3kJ](/tpl/images/0066/4813/c955f.png)



