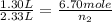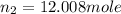Chemistry, 07.09.2019 03:10 allyssaharrisooy50au
Gaseous chlorine is held in two separate containers at identical temperature and pressure. the volume of container 1 is 1.30 l, and it contains 6.70 mol of the gas. the volume of container 2 is 2.33 l. how many moles of the gas are in container 2?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3


You know the right answer?
Gaseous chlorine is held in two separate containers at identical temperature and pressure. the volum...
Questions


Biology, 28.08.2019 11:10

History, 28.08.2019 11:10

Mathematics, 28.08.2019 11:10

Mathematics, 28.08.2019 11:10



Mathematics, 28.08.2019 11:10


English, 28.08.2019 11:10


Biology, 28.08.2019 11:10

Mathematics, 28.08.2019 11:10


Computers and Technology, 28.08.2019 11:10

History, 28.08.2019 11:10

Biology, 28.08.2019 11:10



History, 28.08.2019 11:10

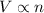
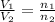
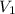 = initial volume of gas in container 1 = 1.30 L
= initial volume of gas in container 1 = 1.30 L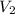 = final volume of gas in container 1 = 2.33 L
= final volume of gas in container 1 = 2.33 L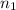 = initial moles of gas in container 2 = 6.70 mole
= initial moles of gas in container 2 = 6.70 mole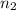 = final moles of gas in container 2 = ?
= final moles of gas in container 2 = ?