
Chemistry, 05.07.2019 19:10 daniiltemkin20
The equilibrium constant for the reaction agbr(s) picture ag+(aq) + br− (aq) is the solubility product constant, ksp = 7.7 × 10−13 at 25°c. calculate δg for the reaction when [ag+] = 1.0 × 10−2 m and [br-] = 1.0 × 10−3 m. is the reaction spontaneous or nonspontaneous at these concentrations?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
The equilibrium constant for the reaction agbr(s) picture ag+(aq) + br− (aq) is the solubility produ...
Questions















Physics, 09.11.2019 07:31


English, 09.11.2019 07:31

English, 09.11.2019 07:31


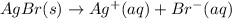
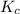 :
:![K_c=\frac{[Ag^+][Br^-]}{[AgCl]}=\frac{[Ag^+][Br^-]}{1}=[Ag^+][Br^-]](/tpl/images/0055/1226/d9269.png)
![K_{sp}=[Ag^+][Br^-]=K_c=7.7\times 10^{-13}](/tpl/images/0055/1226/964b9.png)
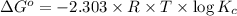
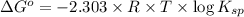
![\Delta G^o=-2.303\times 8.314 J/K mol\times 298 K\times \log[7.7\times 10^{-13}]](/tpl/images/0055/1226/37a54.png)
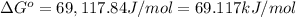
![[Ag^+] = 1.0\times 10^{-2} M](/tpl/images/0055/1226/88952.png) and
and ![[Br^-] = 1.0\times 10^{-3} M](/tpl/images/0055/1226/50b47.png)
![Q=[Ag^+][Br^-]=1.0\times 10^{-2} M\times 1.0\times 10^{-3} M=1.0\times 10^{-5}](/tpl/images/0055/1226/4f48a.png)
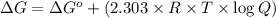
![\Delta G=69.117 kJ/mol+(2.303\times 8.314 Joule/mol K\times 298 K\times \log[1.0\times 10^{-5}])](/tpl/images/0055/1226/947e2.png)
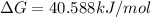
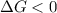 .For reaction to non spontaneous reaction:
.For reaction to non spontaneous reaction: 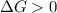 .
.


