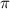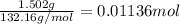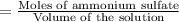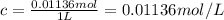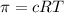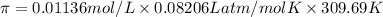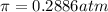Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2
You know the right answer?
Calculate the osmotic pressure of a solution containing 1.502 g of (nh4)2so4 in 1 l at 36.54 degrees...
Questions

Mathematics, 07.05.2020 02:00

Social Studies, 07.05.2020 02:00

Chemistry, 07.05.2020 02:00


Mathematics, 07.05.2020 02:00




Mathematics, 07.05.2020 02:00

Mathematics, 07.05.2020 02:00




Mathematics, 07.05.2020 02:00


Mathematics, 07.05.2020 02:00