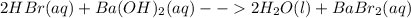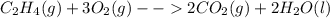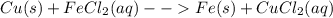Classify each of these reactions. 2hbr(aq)+ba(oh)2(aq)⟶2h2o(l)+babr2( aq) 2hbr(aq)+ba(oh)2(aq)⟶2h2o(l)+babr2( aq) c2h4(g)+3o2(g)⟶2co2(g)+2h2o(l) c2h4(g)+3o2(g)⟶2co2(g)+2h2o(l) cu(s)+fecl2(aq)⟶fe(s)+cucl2(aq) cu(s)+fecl2(aq)⟶fe(s)+cucl2(aq) na2s(aq)+2agno3(aq)⟶2nano3(aq)+ag2s (s) na2s(aq)+2agno3(aq)⟶2nano3(aq)+ag2s (s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3


Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Classify each of these reactions. 2hbr(aq)+ba(oh)2(aq)⟶2h2o(l)+babr2( aq) 2hbr(aq)+ba(oh)2(aq)⟶2h2o(...
Questions

Biology, 16.12.2019 19:31



English, 16.12.2019 19:31


History, 16.12.2019 19:31

English, 16.12.2019 19:31


Mathematics, 16.12.2019 19:31


Mathematics, 16.12.2019 19:31

Biology, 16.12.2019 19:31

Mathematics, 16.12.2019 19:31



Physics, 16.12.2019 19:31

Mathematics, 16.12.2019 19:31

Mathematics, 16.12.2019 19:31