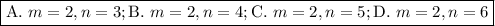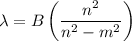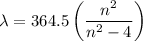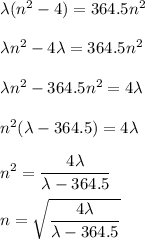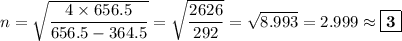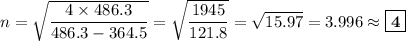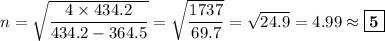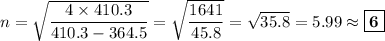Determine the balmer formula n and m values for the wavelength 656.5 nm. possible choices: m= 1 n= 2 m= 2 n= 3 m= 3 n= 4 m= 2 n= 5 part b determine the balmer formula n and m values for the wavelength 486.3 nm. possible choices: m= 1 n=2 m= 2 n=3 m= 1 n=4 m= 2 n=4 part c determine the balmer formula n and m values for the wavelength 434.2 nm. possible choices: m= 1 n= 4 m= 2 n= 4 m= 3 n= 4 m= 2 n= 5 part d determine the balmer formula n and m values for the wavelength 410.3 nm. possible choices: m= 2 n= 4 m= 2 n= 5 m= 3 n= 4 m= 2 n= 6

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1
You know the right answer?
Determine the balmer formula n and m values for the wavelength 656.5 nm. possible choices: m= 1 n=...
Questions


Computers and Technology, 18.10.2019 04:30




Social Studies, 18.10.2019 04:30

History, 18.10.2019 04:30

History, 18.10.2019 04:30


Social Studies, 18.10.2019 04:30









Computers and Technology, 18.10.2019 04:30