
Chemistry, 28.06.2019 03:00 aidentrooper8629
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric acid. if the percent yield of the reaction was 82.15%, what was the actual amount of calcium chloride formed? caco3 + hcl → cacl2 + co2 + h2o 105.3 grams 101.1 grams 95.6 grams 86.5 grams

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric ac...
Questions


History, 03.09.2020 09:01

Business, 03.09.2020 09:01


Mathematics, 03.09.2020 09:01




English, 03.09.2020 09:01

Chemistry, 03.09.2020 09:01

World Languages, 03.09.2020 09:01


Social Studies, 03.09.2020 09:01

History, 03.09.2020 09:01


English, 03.09.2020 09:01


Computers and Technology, 03.09.2020 09:01

Mathematics, 03.09.2020 09:01

English, 03.09.2020 09:01

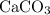 ?
?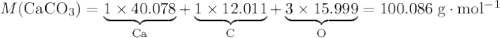 .
.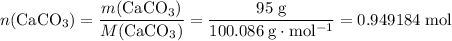 .
.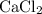 will be produced?
will be produced?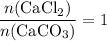 .
.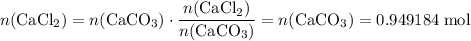 .
. of
of 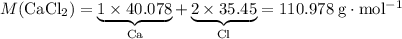 .
.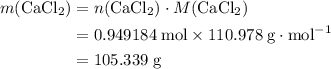 .
.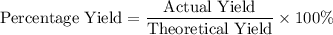 .
. .
.

