
Chemistry, 22.12.2019 12:31 icecreamisgood4u
One of the main components of pearls is calcium carbonate (caco3). if pearls are put into an acidic solution, they dissolve: caco3(s) + 2hcl(aq) ➡ cacl2(aq) + h2o(l) + co2(g). how many moles of caco3 can be dissolved in 0.0250 moles of hydrochloric acid?
0.0125 moles
0.0500 moles
2.50 moles
4.50 moles

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
One of the main components of pearls is calcium carbonate (caco3). if pearls are put into an acidic...
Questions

Computers and Technology, 02.10.2019 20:10


Chemistry, 02.10.2019 20:10



Computers and Technology, 02.10.2019 20:10

Chemistry, 02.10.2019 20:10

Chemistry, 02.10.2019 20:10

Chemistry, 02.10.2019 20:10

Chemistry, 02.10.2019 20:10




Chemistry, 02.10.2019 20:10

Chemistry, 02.10.2019 20:10

Chemistry, 02.10.2019 20:10


Chemistry, 02.10.2019 20:10


Chemistry, 02.10.2019 20:10

![CaCO_3(s)+2HCl(aq)]\rightarrow CaCl_2(aq)+H_2O(l)+CO_2(g)](/tpl/images/0429/7730/2251f.png)
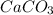
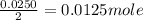 of
of 

