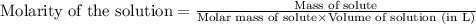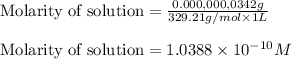Chemistry, 02.10.2019 20:10 mmagee2020
What is the molarity of .342 grams of adenosine 3'5' cyclic monophosphate (camp) in one lite

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 08:40
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3
You know the right answer?
What is the molarity of .342 grams of adenosine 3'5' cyclic monophosphate (camp) in one lite...
Questions


Social Studies, 15.12.2021 23:20


Mathematics, 15.12.2021 23:20

Mathematics, 15.12.2021 23:20

English, 15.12.2021 23:20

Mathematics, 15.12.2021 23:20

Physics, 15.12.2021 23:20

Mathematics, 15.12.2021 23:20


Biology, 15.12.2021 23:20


History, 15.12.2021 23:20






Arts, 15.12.2021 23:20