
Chemistry, 08.10.2019 02:30 polyanskiymichael
Read the chemical equation. n2 + 3h2 → 2nh3 using the volume ratio, determine how many liters of nh3 is produced if 1.2 liters of h2 reacts with an excess of n2, if all measurements are taken at the same temperature and pressure? 1.8 liters 1.5 liters 0.90 liters 0.80 liters

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

You know the right answer?
Read the chemical equation. n2 + 3h2 → 2nh3 using the volume ratio, determine how many liters of nh3...
Questions


Mathematics, 05.01.2021 03:40

Biology, 05.01.2021 03:40


Mathematics, 05.01.2021 03:40


SAT, 05.01.2021 03:40

Mathematics, 05.01.2021 03:40


Mathematics, 05.01.2021 03:40


Biology, 05.01.2021 03:40

Mathematics, 05.01.2021 03:40





History, 05.01.2021 03:40

Mathematics, 05.01.2021 03:40

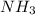 produced will be, 0.8 liters.
produced will be, 0.8 liters.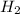 = 1.2 L
= 1.2 L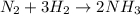
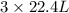 volume of
volume of 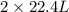 volume of
volume of 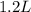 volume of
volume of 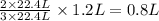 volume of
volume of 


