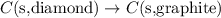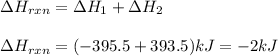Chemistry, 11.01.2020 03:31 mallorybranham
Given the equations below, which description applies to the conversion of diamond to graphite? c(s, diamond) + o2 (g) → co2 (g), ∆h = –395.4 kj co2 (g) → c(s, graphite) + o2 (g), ∆h = 393.5 kj explain your answer
a) energy is created during the process.
b) heat is neither released nor absorbed during the process.
c) heat is released during the process.
d) the process is endothermic.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1


Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Given the equations below, which description applies to the conversion of diamond to graphite? c(s,...
Questions








Social Studies, 03.01.2020 23:31

Arts, 03.01.2020 23:31






Computers and Technology, 03.01.2020 23:31





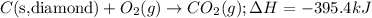 ....(1)
....(1)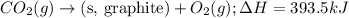 ....(2)
....(2)