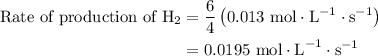Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
For the reaction: 4ph3(g) → p4(g) + 6h2(g) about 0.065 mol/s of ph3 is consumed in a 5.0 l flask. w...
Questions

Mathematics, 27.01.2021 15:40


Mathematics, 27.01.2021 15:40


Mathematics, 27.01.2021 15:50

Biology, 27.01.2021 15:50

History, 27.01.2021 15:50

Mathematics, 27.01.2021 15:50

Arts, 27.01.2021 15:50



History, 27.01.2021 15:50

Mathematics, 27.01.2021 15:50

Mathematics, 27.01.2021 15:50

Physics, 27.01.2021 15:50

Physics, 27.01.2021 15:50

Mathematics, 27.01.2021 15:50



Chemistry, 27.01.2021 15:50

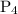 and
and 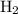 are
are 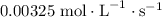 and
and 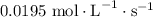 respectively.
respectively.
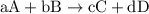
![{\text{Rate of reaction}} = - \dfrac{1}{{\text{a}}}\dfrac{{d\left[ {\text{A}} \right]}}{{dt}} = - \dfrac{1}{{\text{b}}}\dfrac{{d\left[ {\text{B}} \right]}}{{dt}} = \dfrac{1}{{\text{c}}}\dfrac{{d\left[ {\text{C}} \right]}}{{dt}} = \dfrac{1}{{\text{d}}}\dfrac{{d\left[ {\text{D}} \right]}}{{dt}}](/tpl/images/0015/9793/1d76f.png)
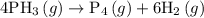
![{\text{Rate of reaction}}= - \dfrac{1}{4}\dfrac{{d\left[ {{\text{P}}{{\text{H}}_{\text{3}}}} \right]}}{{dt}} = \dfrac{{d\left[ {{{\text{P}}_{\text{4}}}} \right]}}{{dt}} = \dfrac{1}{6}\dfrac{{d\left[ {{{\text{H}}_2}} \right]}}{{dt}}](/tpl/images/0015/9793/a7105.png) …… (1)
…… (1)
![\dfrac{{d\left[ {{\text{P}}{{\text{H}}_{\text{3}}}} \right]}}{{dt}}](/tpl/images/0015/9793/9005a.png) is the rate of consumption of
is the rate of consumption of 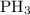 .
.
![\dfrac{{d\left[ {{{\text{P}}_{\text{4}}}} \right]}}{{dt}}](/tpl/images/0015/9793/a3838.png) is the rate of formation of
is the rate of formation of 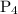 .
.
![\dfrac{{d\left[ {{{\text{H}}_2}} \right]}}{{dt}}](/tpl/images/0015/9793/f5e79.png) is the rate of formation of
is the rate of formation of 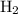 .
.
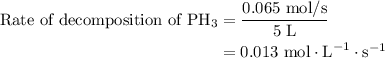
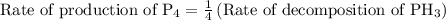 …… (2)
…… (2)
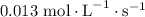 . Substitute this value in equation (2).
. Substitute this value in equation (2).
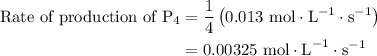
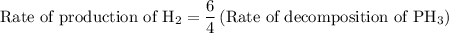 …… (3)
…… (3)